Describe in Words the Reaction Represented by the Equation
Use the principle of conservation of matter to explain why the equation in question 4 is balanced. HBraq KOHaq KBraq HOO b.
Ch104 Chapter 5 Chemical Reactions Chemistry
Reactants Product Substances.

. Chemical equations reactions materials conserving matter reaction 8 2 how do we represent siyavula chemistry a you what are detailed explanation examples described by writing balanced equation lessons and solutions types of with chapter 1 page 7 drgp institute Chemical Equations Reactions Materials Conserving Matter Reaction 8 2 How Do We Represent. In each case the mass of the flask and its contents was the same after the reaction as it was before the reaction occurred showing that mass had not been created or destroyed in the reaction. Chemical equations were first formulated by the French chemist Jean Beguin in the year 1615.
The reactants are typically written on the left side of the equation and. For example melting sublimation evaporation and condensation can be represented as follows. 6 CO2 6 H2O light energy C6H12O6 glucose 6 O2 But this balance is in fact broken down into two successive stages.
If you were to describe a chemical reaction in a sentence it would be quite long. Describe in words the chemical composition of the molecules involved and the reaction represented by the equation. The Calvin cycle dark phase.
2 KBraq Clug 2 KClaq Br0 C. In chemical equations reactants and products are represented by chemical symbols and formulas. Write a balanced net ionic equation for the overall reaction represented by the cell notation below.
The general form of a chemical equation is. In these equations s stands for solid l for liquid l and g for gas. AgNOlaq KCagAgCls KNO aq HBraq KOHaq KBraq HO This problem has been solved.
2NO2 2O2 N2. 2W aq X aq 2Y aq K c 5 10 4 a Write the mathematical expression for the equilibrium constant. The word equation indicates that the.
B Using concentrations of 1 M make up two sets of concentrations that describe a. 2 H2 O2 2 H2O Answers. 2 H 2 O 2 2 H 2 O.
The reaction of aqueous ironII sulfate and aqueous barium nitrate is represented by the balanced net ionic equation. AgNOaq KClaq - AgCls KNO3aq a. Answer questions 4 and 5 on a separate sheet of paper.
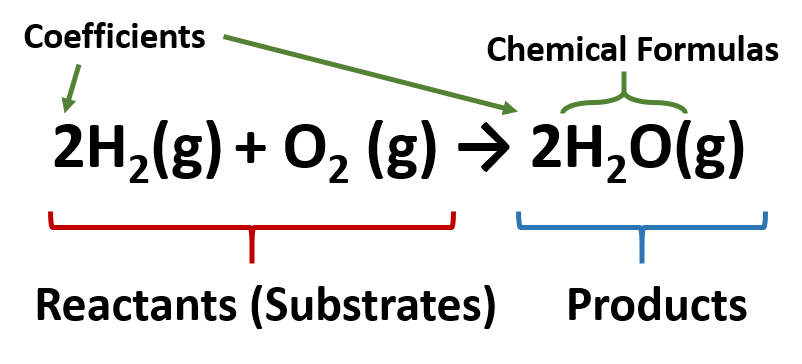
Magnesium Oxygen Magnesium oxide. The reactants in a chemical equation are the substances that begin the reaction and the products are the substances that are produced in the reaction. A chemical equation is a shorthand way to sum up what occurs in a chemical reaction.
Chemical reactions can be represented on paper with the help of chemical equations an example for which is represented below for the reaction between hydrogen gas and oxygen gas to form water. Numbers called coefficients are placed in front of the symbols and formulas to show how much of each substance is involved in the reaction. Describe in words the reaction represented by the equation.
Decomposition or replacement reaction. 6 CO2 chemical energy C6H12O6 6 H2O. For example the reaction between magnesium and oxygen to give magnesium oxide can be represented as follows.
To express it in a shorter form we can write it as a word-equation. SO42-aq Ba2aq BaSO4s Give the balanced formula equation for the reaction. 12 H2O light 6 O2 chemical energy.
Describe in words the reaction represented by the equation and include a description of the composition of each kind of molecule. The new substance is magnesium oxide which is formed as a product during the reaction. A balanced equation has the same number of each type of atom on.
300 Describe in words the chemical reaction represented by the following chemical equation. The response word equation is above-Magnesium Oxygen Respondent Magnesium Oxide Product11 The substances that cause a chemical change in the reaction 11 magnesium and oxygen are the reaction substances. Chemical equations must be balanced.
Here is the reaction of photosynthesis. Photochemical reactions clear phase. Include a description of the composition of each kind of molecule.
An equation can be used to describe a physical reaction which involves a change of states. Describe in words the reactions represented by the following chemical equations. 2H202-- 2H2O 4 molecule Hydrogens reacts to 2 molecule Oxygen to yield 2 molecules of water.
2H 2 O 2 2H 2 O. Describe in words the reactions represented by the following chemical equations. A reaction is represented by this equation.

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab

What Are Chemical Equations Detailed Explanation Examples

Main Chemical Reaction Equations Download Scientific Diagram
No comments for "Describe in Words the Reaction Represented by the Equation"
Post a Comment