The Term Polar Is Used to Describe Molecules Because
Polar molecules occur when two atoms do not share electrons equally in a covalent bond. 1 Answer zhirou May 27 2018 When a molecule is polar it means that it has positive and negative ends.
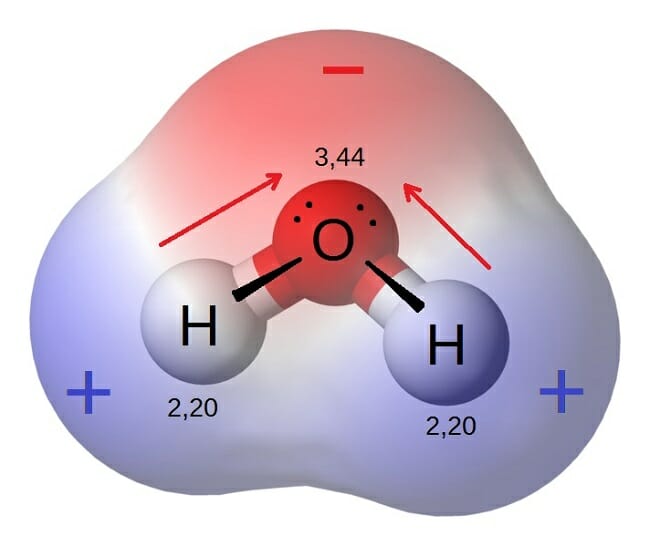
Polar Molecule Definition And Examples Biology Dictionary
Polar molecule Middle School Level noun a molecule in which the centroid of the positive charges is different from the centroid of the negative charges.
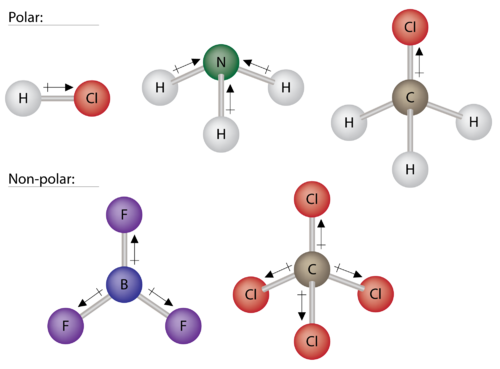
. By definition polarity is basically the status of having poles In a molecule this means that the molecule has poles of positive and negative chargealso known as a separation of charge. This happens when there is a difference between the electronegativity values of each atom. C there are at least two distinct ends of the molecule regarding electron position and.
Polar Molecules. Silicon Si has an electronegativity of 18. A water molecule is polar because.
No For a molecule to be considered polar what must it contain. The more polar the bond is Just because a molecule posseses polar bonds does it mean the molecule as a whole will be polar. Predict the structures of small molecules using valence shell electron pair repulsion VSEPR theory Explain the concepts of polar covalent bonds and molecular polarity Assess the polarity of a molecule based on its bonding and structure Thus far we have used two-dimensional Lewis structures to represent molecules.
Do you have the grammar chops to know when to use. The term polar is used to describe molecules because A polar covalent molecules are found in colder climates. A polar molecule is a type of molecule that has a separation of electric charge where one side of the molecule is positively charge and the other side is negatively charged.
B polar covalent molecules were first discovered in polar bears. The characteristic of bonding A to T and G to C is called complementary _____. 101 The term polar is used to describe molecules because A polar covalent molecules are found in colder climates.
B polar covalent molecules were first discovered in polar bears. QUIZ QUIZ YOURSELF ON HAS VS. A polar molecule is characterized by the uneven distribution of the electrons that form the covalent bonds between each atom in the molecule resulting in a slightly positively charged side and a slightly negatively charged side.
The polarity of a molecule depends upon the electronegativity of the constituent elements of that molecule. Water or H2O is. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared ie bonding electrons for eight total electrons matching the number of valence electrons in the noble gas argon.
Polarity Define Polarity A state or a condition of an atom or a molecule having positive and also negative charges especially in case of magnetic or an electrical poles Polarity in general refers to the physical properties of compounds such as boiling point melting points and their solubilities. Hydrogen H has an electronegativity of 21. Match each level of protein structure with its description.
Because of this one DNA strand can be used as a template to make a. A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed. However in general terms we can use the terms polar and dipolar interchangeably because both these terms describe a single molecule having two opposite ends.
A dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. Must contain at least one polar bond and it must have two distinct regions of opposite charge. For example water is.
There are at least two distinct ends of the molecule regarding electron position and the resulting charge. C there are at least two distinct ends of the molecule regarding electron position and the resulting charge. This occurs because of the differences in electronegativity between atoms of different elements.
Elements that differ greatly in electronegativities will exert unequal attractions on. This occurs in molecules that are asymmetric along at least one axis when one side contains atoms with a. Air can dissolve easily in water.
A polar molecule is a particle consisting of two or more covalently bonded atoms with an asymmetric distribution of charges. This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative. Since the bonding atoms are identical Cl 2 also features a pure covalent bond.
The key difference between polar and dipolar molecules is that polar molecules have two opposite ends with opposite electrical charges whereas dipolar molecules have two poles. The single common feature of these molecules is that they are only slightly soluble in water. Polarity in chemistry refers to the unequal attraction of electrons in elements of a compound resulting in a molecule with a negatively charged end and a positively charged end.
The term _____ is used to describe a diverse group of non-polar hydrophobic organic molecules. An extreme difference forms an. ClCl Cl2 Cl Cl Cl 2.
Terms in this set 16 True or False. The term polar is used to describe molecules because.
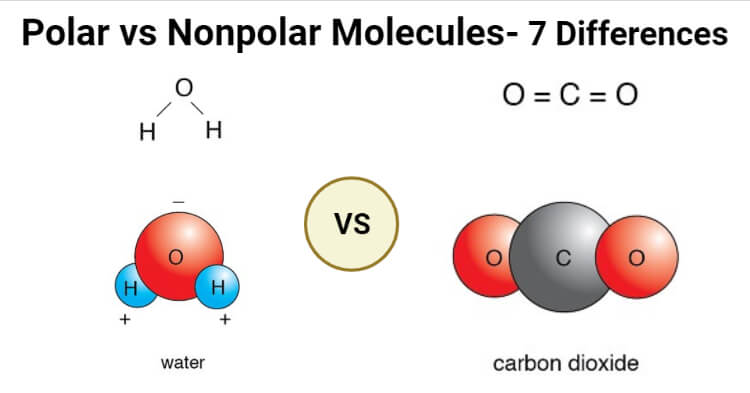
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples

Polar Molecules Chemistry For Non Majors

Why Is Water A Polar Molecule Water Molecule Molecules Polarity Of Water
No comments for "The Term Polar Is Used to Describe Molecules Because"
Post a Comment